[ad_1]
The clinical trial will evaluate the safety, immunogenicity and efficacy of the vaccine. It is being developed by a Spanish biopharmaceutical player. Biofabri in collaboration with Bharat Biotech.
Sources said Bharat Biotech will have exclusive global manufacturing rights for the live attenuated vaccine being developed for newborns, adolescents and adults.
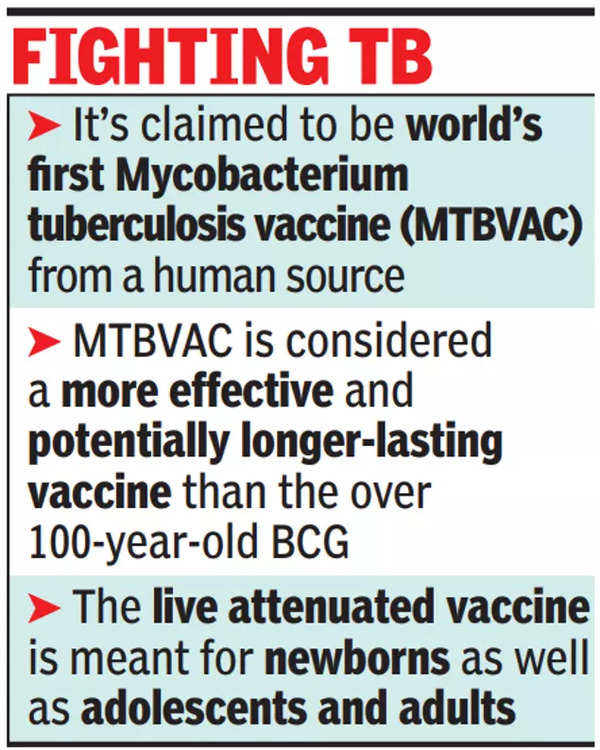
MTBVAC is being developed as a more effective and potentially longer lasting vaccine than the BCG vaccine, which is more than 100 years old. It will be for newborns, as well as for the prevention of tuberculosis in adults and adolescents for whom there is currently no effective vaccine, sources said.
The existing BCG vaccine is an attenuated variant of the bovine tuberculosis pathogen and has limited effect on pulmonary tuberculosis, which is responsible for the transmission of the disease.
Studying the safety, immunogenicity and efficacy of MTBVAC in the most populated country in the world and with the highest number of tuberculosis cases is key to continuing the advancement of the vaccine, which has been the subject of more than three decades of research.
The general director of Biofabri, Esteban Rodríguez, described it as a “giant step to carry out tests on adults and adolescents in the country where 28% of tuberculosis cases in the world accumulate.”
Dr. Krishna Ella, CEO of Bharat Biotech, called the clinical trials in India a big step in the goal of developing tuberculosis vaccines to prevent this potentially deadly disease in adults and adolescents: “Our search for a more effective against tuberculosis received great support. “There is a lot of momentum today, with clinical trials in India.”
MTBVAC is currently the only tuberculosis vaccine is undergoing clinical trials based on a genetically modified form of the pathogen, Mycobacterium tuberculosis, which, unlike BCG, contains all the antigens present in strains that infect humans.
The vaccine was developed in the laboratory of the University of Zaragoza in collaboration with Dr. Brigitte Gicquel from the Pasteur Institute in Paris. Biofabri is the industrial partner of the University of Zaragoza.
The vaccine recently completed a Phase 2 dose-finding trial. A controlled, double-blind Phase 3 clinical trial in newborns began in 2023 in South Africa, Madagascar and Senegal to compare it with the only tuberculosis vaccine in use, the BCG vaccine.

