[ad_1]

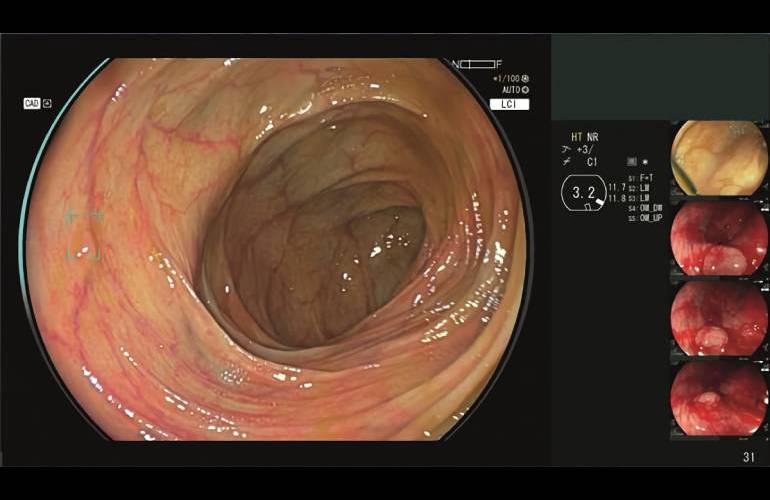
fujifilm announced today that the FDA has granted 510(k) clearance for its CAD Eye AI-based detection system for endoscopic imaging.
CAD Eye allows real-time detection of colonic mucosal lesions, such as polyps and adenomas, during colonoscopy procedures. It helps endoscopists detect and remove precancerous lesions, regardless of size, shape and color. The system enters a market currently dominated by Medtronic and its GI Genius system.
GI Genius, a computer-assisted polyp detection system powered by AIwas first available in the United States in 2021. Read more about Medtronic AI-related efforts for GI Genius here.
Both tools were designed to help endoscopists detect colonic mucosal lesions. CAD Eye works with both white light images and linked color images (LCI). Fujifilm included an improved viewing mode to differentiate the red color spectrum, improving visualization of the mucosa. Anthony Borrelli, executive director of endoscopy marketing and products at Fujifilm Healthcare Americas Corporation, said mass device that LCI capabilities, in addition to HD white light, are a key differentiator over the competitive GI Genius.
The Fujifilm system features a compatible expansion unit (Fujifilm EX-1) and endoscopy support software (EW10-EC02). CAD Eye is based on the company’s Eluxeo endoscopic imaging system, with AI image processing for integration with the system’s processor and endoscope.
The company plans to commercialize CAD Eye this spring after completing a limited market evaluation.
“Every day, we pride ourselves on delivering what we believe are the highest quality images and optics, giving endoscopists the tools they need to combat this public health issue.” saying Tai Fujita, general manager, endoscopy, Fujifilm Healthcare Americas Corporation. “Today, we are delighted to take this a step further with the introduction of CAD Eye, which has the potential to dramatically improve the quality of colonoscopy.”
Other possible differentiators
According to Borelli, Fujifilm’s in-house development of the Eye CAD system and artificial intelligence training also separate the technology from the rest. The developers trained the system 100% with images captured by Fujifilm’s endoscopic imaging team.
Borelli said: “Fujifilm believes this is a great strength, as the company is known for its excellent optics and image quality, especially in its endoscopic imaging systems, which we believe strengthens the CAD Eye product.”
Additionally, it offers seamless integration into the physician’s workflow. Clinicians can turn CAD Eye on and off on the endoscopic handle, without needing to turn toward the equipment tower, take their eyes off the patient, or ask for help.
“Customer feedback indicates that this is highly impactful to their workflows and a strong differentiator,” Borelli added.
More about the Fujifilm CAD Eye system
The company developed CAD Eye using deep learning technology at its global AI technology center based in Tokyo. Fujifilm validated the system using histologically confirmed polyps on clinical images.
According to a press release, the technology makes it possible to detect injuries that could easily go unnoticed. This includes flat lesions, those in the corner of the endoscopic view, and multiple lesions present in a single frame. When CAD Eye identifies a suspicious polyp, the doctor automatically receives visual and auditory alerts. Visual cues overlap but do not interfere with clinical images within the existing workflow.
Fujifilm said the studies demonstrated CAD Eye’s ability to make significant advances in the detection and diagnosis of colorectal cancer. The system detects significantly more adenomas during screening and surveillance compared to conventional high-definition colonoscopy without AI assistance. Furthermore, that occurs without any increase in procedure time.
Additionally, Fujifilm detected 17% more adenomas by colonoscopy compared to conventional high-definition colonoscopy. The system detects colorectal neoplastic lesions at a level comparable to that of an expert and superior to that of a novice, the company stated.
“As a gastroenterologist dedicated to patient care and safety, I am encouraged by the FDA’s approval of Fujifilm’s AI CAD polyp detection algorithm. “This advancement not only allows for early detection of precancerous lesions that can lead to colorectal cancer, but also significantly reduces the risk of missed lesions, improving our accuracy and patient outcomes,” DR said. Sravanthi Parasa of the Swedish Medical Center. “It marks a fundamental advance in our ability to safeguard patient health, reinforcing our commitment to adopting technological innovations in gastrointestinal healthcare.”
[ad_2]
Source link